- Home
- Lab Supplies
- Lab Chemicals
.....Read More

Chemicals beginning with A

Chemicals beginning with B

Chemicals beginning with C

Chemicals beginning with D

Chemicals beginning with E

Chemicals beginning with F

Chemicals beginning with G

Chemicals beginning with H
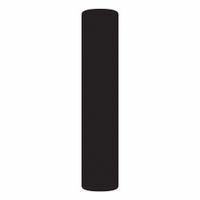
Chemicals beginning with I

Chemicals beginning with J

Chemicals beginning with K
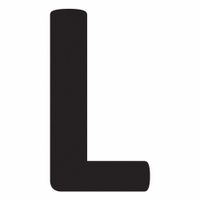
Chemicals beginning with L

Chemicals beginning with M

Chemicals beginning with N
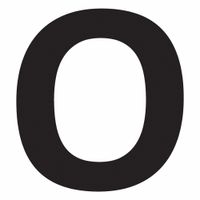
Chemicals beginning with O

Chemicals beginning with P

Chemicals beginning with Q

Chemicals beginning with R

Chemicals beginning with S
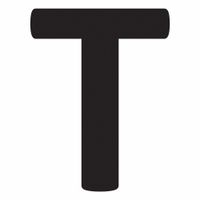
Chemicals beginning with T

Chemicals beginning with U

Chemicals beginning with V

Chemicals beginning with W

Chemicals beginning with X

Chemicals beginning with Y

Chemicals beginning with Z
Frequently Asked Questions
What are lab chemical grades and their meanings?
How do I choose the right chemical grade for my application?
What is the difference between ACS and reagent grade chemicals?
Why is chemical purity important in pharmaceutical development?
How are lab chemicals tested for purity?
What organizations set standards for chemical grades?
Can I use industrial-grade chemicals for research purposes?
What safety precautions should be taken when handling lab chemicals?
How do I store lab chemicals properly?
What are the environmental impacts of using lab chemicals?